R&D | KIMS developed waste alkali-based anion exchange membrane water electrolysis technology
Page info
Date22-12-16 16:05 Hit290Link
Contents
Producing hydrogen from wasted water! |
A research team led by Dr. Juchan Yang and Dr. Sung Mook Choi of the Department of Green Hydrogen Materials at the Korea Institute of Materials Science (KIMS), a government-funded research institute under the Ministry of Science and ICT, succeeded in developing a high-performance non-precious metal catalyst/electrode material. The researchers also developed anion exchange membrane water electrolysis technology using a non-noble metal-based waste alkali water electrolysis system. It is the first case in Korea.
Anion exchange membrane water electrolysis is an ideal technology that combines the advantages of existing non-noble metal-based electrodes of alkaline water electrolysis technology and proton exchange membrane water electrolysis which is easily operated for its simplicity. The technology is a next-generation water electrolysis system that can improve the economic feasibility of green hydrogen production and obtain high-purity hydrogen.
Water electrolysis producing green hydrogen has so far used electrolytes in purified water, which requires an average of about 9 tons of purified water to produce 1 ton of hydrogen through water electrolysis. To obtain 9 tons of purified water, about twice as much water is required. Since the production of green hydrogen requires not only electricity for electrolysis but also a huge amount of water, the cost of water supply is also an important factor in determining price competitiveness.
Waste alkali means strong alkaline waste with a pH of 12.5 or higher, and the discarded amount is increasing every year due to the surge in demand for semiconductors and the growth of the electronics industry. The researchers at KIMS applied the key source materials and parts of water electrolysis, which directly uses waste alkali as an electrolyte, to a unit cell that mimics a commercial system.
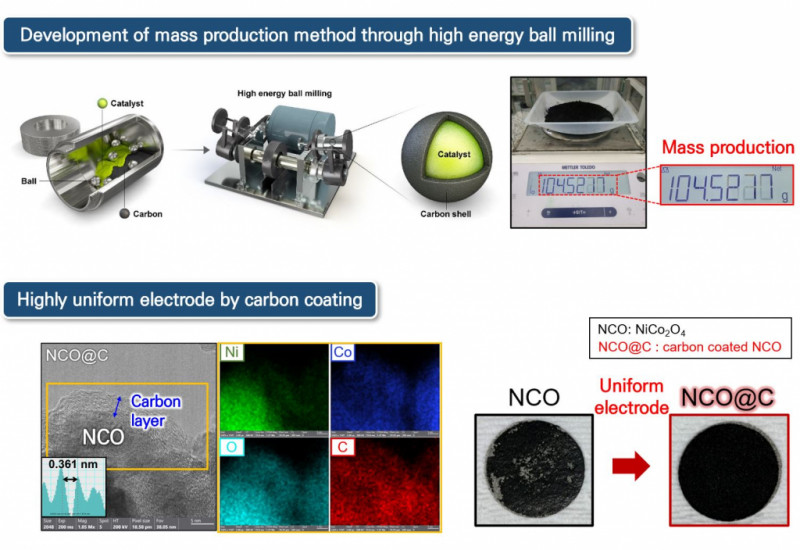
First of all, the research team developed a composite catalyst in which a high-conductivity carbon layer is uniformly coated on the catalyst surface in order to improve both activity and durability of a non-noble metal catalyst. The catalyst showed high uniformity in the electrodeization process and was evaluated to have about 2.8 times better activity and high durability than conventional electrodes in the unit cell evaluation of the anion exchange membrane water electrolysis. As a result of applying this technology to a waste alkali electrolyte-based water electrolysis unit cell, it achieved a high current density of 1,420 mA/cm2 (non-noble metal and 2V applied) and showed improved durability even at a current density that can smoothly produce hydrogen. In conclusion, researchers revealed that the increased electrolyte ionic conductivity improved the water electrolysis performance by analyzing metal ions in waste alkali.
Dr. Juchan Yang, the principal investigator and a senior researcher at KIMS said, "Using waste alkali electrolytes makes it easy to recycle and treat waste alkali, and it is possible to drastically reduce the amount and cost of using high-concentration pure alkali salt electrolyte.”Dr. Sung Mook Choi, a project leader and the Director of Green Hydrogen Materials Division, said, “When this technology is commercialized, it can be immediately applied to the existing alkali-based water electrolysis system, enabling mass production and entering the hydrogen production market.”
This research was carried out as a major project of KIMS (titled development of durable electrode and evaluation for anion exchange membrane water electrolysis for production of green hydrogen) and a material and part R&D project of the Ministry of Trade, Industry and Energy (titled development of high-performance catalytic electrodes and parts technology on 2.5kW-class AEM water electrolysis using waste-alkali solution for hydrogen production) and funded by the Ministry of Science and ICT. The research outcomes were published in the Journal of Materials Chemistry A (IF=14.511, 1st author: Ph.D. student Jae-yeop Jeong, Dr. Yoo Sei Park (professor at Chungbuk National University)) on November 10. The research team is conducting follow-up research for the application and commercialization of the waste alkali water electrolysis stack system through large-area uniform electrodeization.